

In the 107 patients with squamous NSCLC receiving nivolumab plus chemotherapy and 105 on chemotherapy, median OS was 18.3 versus 12.0 months (HR 0.69 95% CI, 0.50-0.97) and 12-month OS rates were 66.1% versus 48.5%, respectively. The impact in OS was more evident in patients with squamous histology and in the overall population. A total of 156 (57.8%) and 164 (60.1%) OS events had occurred with nivolumab/chemotherapy versus chemotherapy and the one-year OS rates were 67.3% versus 59.2%, respectively. With minimum follow-up of 19.5 months in the groups of 270 and 273 patients with non-squamous NSCLC who were treated with the nivolumab combination and chemotherapy, respectively, no statistically significant improvement in OS was seen with nivolumab plus chemotherapy over chemotherapy alone median OS was 18.8 versus 15.6 months with the respective treatments (hazard ratio 0.86 95,62% confidence interval, 0.69–1.08 p = 0.1859). The primary endpoint was OS with nivolumab plus chemotherapy as compared with chemotherapy in patients with non-squamous NSCLC and a secondary hierarchical endpoint was OS in all randomised patients. Pemetrexed maintenance was available to patients with non-squamous NSCLC. The patients were stratified by histology (squamous versus non-squamous sex, and PD-L1 expression status (<1% versus ≥1%).
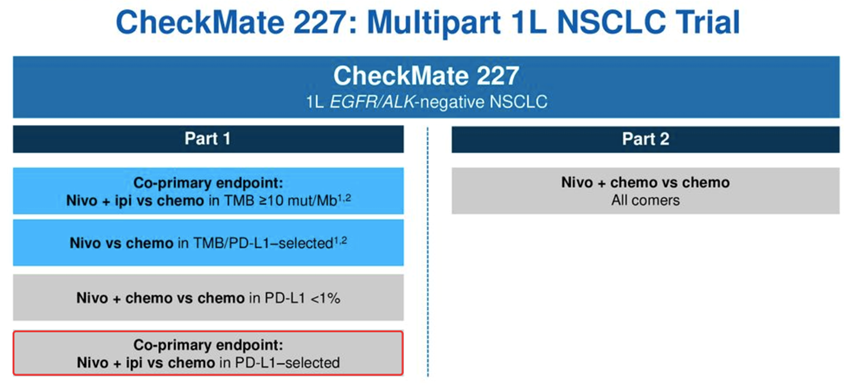
Baseline characteristics between treatment arms were generally balanced. The patients were required to have no sensitising EGFR/ALK alterations.įollowing 1:1 randomisation, 377 patients received nivolumab at 360 mg for up to 2 years plus histology-based chemotherapy for up to 4 cycles, progression, or unacceptable toxicity, and 378 patients received the same chemotherapy regimen. Luiz Paz-Ares of the Medical Oncology Department, University Hospital 12 de Octubre and Universidad Complutense and CiberOnc in Madrid, Spain presented findings from part 2 of the final analysis of data from the multi-part, randomised, open-label, phase III CheckMate 227 (NCT02477826) study of first-line nivolumab plus chemotherapy versus chemotherapy alone.ĬheckMate 227enrolled 755 patients with chemotherapy-naive, ECOG performance status 0–1, stage IV or recurrent NSCLC.
Checkmate 227 trial#
These findings from the phase III CheckMate 227 trial were presented at the ESMO Immuno-Oncology Congress 2019 in Geneva, Switzerland (11-14 December). Additionally, all groups of patients treated with nivolumab/chemotherapy showed prolonged progression-free survival (PFS) and improved objective response rates (ORR) over chemotherapy alone. Although overall survival (OS) in patients with non-squamous non-small cell lung cancer (NSCLC) with first-line nivolumab plus chemotherapy versus chemotherapy did not reach statistical significance and the primary endpoint was not met, groups of patients with squamous NSCLC and all randomised patients did show improved OS with the combination treatment.


 0 kommentar(er)
0 kommentar(er)
